BioterrorismMarburg drug shows promise
Marburg hemorrhagic fever is a severe and highly lethal disease with no effective treatments, and it has been classified as a Category A bioterrorism agent by the Centers for Disease Control and Prevention (CDC). Sarepta Therapeutics announced positive results from a non-human primate study of AVI-7288, the company’s lead drug candidate for the treatment of Marburg virus infection.
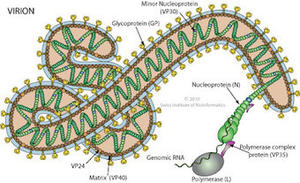
Structure of the Marburg virus // Source: expasy.org
Sarepta Therapeutics, a developer of RNA-based therapeutics, the other day announced positive results from a non-human primate study of AVI-7288, the company’s lead drug candidate for the treatment of Marburg virus infection. The data showed that intramuscular administration of AVI-7288 resulted in survival rates up to 100 percent in treated subjects, similar to efficacy observed in previous studies that evaluated the drug when administered by intravenous injection.
Marburg hemorrhagic fever is a severe and highly lethal disease with no effective treatments, and it has been classified as a Category A bioterrorism agent by the Centers for Disease Control and Prevention (CDC).
“These data reinforce the strong efficacy of AVI-7288, while showing that the drug can be delivered via a convenient intramuscular injection,” said Chris Garabedian, president and chief executive officer of Sarepta Therapeutics. “This alternative delivery method to the intravenous route has the potential to greatly enhance the practical utility of AVI-7288 in a mass casualty situation and also serves as a model for delivery of our rapidly adaptable platform for other therapeutic applications.”
Sarepta is developing AVI-7288 under a U.S. Department of Defense (DoD) contract managed by the Joint Project Manager Transformational Medical Technologies (JPM-TMT) Project Management Office, a component of the Joint Program Executive Office for Chemical and Biological Defense (JPEO-CBD). Under the contract, Sarepta initiated the study in non-human primates to evaluate the tolerability, pharmacokinetics, and efficacy of AVI-7288 through intramuscular administration. The study included four cohorts in which subjects received daily treatments of AVI-7288 ranging from 7.5 to 30 mg/kg or a placebo after exposure to the virus.
In the study, intramuscular injections of AVI-7288 were well tolerated. Efficacy results showed a high degree of survival between 83 and 100 percent in each of the three treatment groups. No subjects survived in the placebo-treated control group.
Sarepta says that under a separate contract with JPM-TMT, it is developing an intravenous formulation of AVI-7288, which has demonstrated similar survival rates even when the drug is administered up to four days after exposure to the Marburg virus.
The work is a collaborative effort between Sarepta and scientists at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), the DoD’s leading medical research laboratory for biological defense, which has the DoD’s only maximum containment, or Biosafety Level 4, capability.
About Marburg virus
Marburg hemorrhagic fever is a severe and potentially fatal disease in humans first recognized in 1967. It is caused by an RNA virus of the Filoviridae family and is understood to be endemic to Africa. The Marburg virus is classified as a Category A bioterrorism agent by the Centers for Disease Control and Prevention, or CDC, and is a material threat to national security and public health as determined by the Secretary of Homeland Security in 2006. Onset of the disease is often sudden, and the symptoms include fever, chills, nausea, vomiting, chest pain, and diarrhea. Increasingly severe symptoms may also include massive hemorrhaging and multiple organ dysfunctions. There are currently no treatments for Marburg virus infection beyond supportive care.
