AnthraxDeveloping new anthrax vaccine
The Texas A&M Center for Innovation in Advanced Development and Manufacturing (CIADM) will produce an intranasal anthrax vaccine candidate under a task order issued by the U.S. Department of Health and Human Services (HHS). This is the first task order issued to the Texas A&M center and will enhance protection from anthrax disease. The Texas A&M facility is one of three CIADMs — and the only academically-based center — established as public-private partnerships with BARDA in 2012 to enhance the nation’s emergency preparedness against emerging infectious diseases.
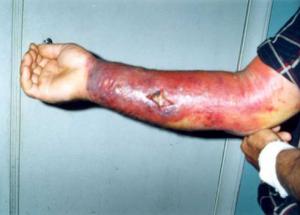
Skin reaction to anthrax // Source: wikipedia.org
The Texas A&M Center for Innovation in Advanced Development and Manufacturing (CIADM) will produce an intranasal anthrax vaccine candidate under a task order issued by the U.S. Department of Health and Human Services (HHS). This is the first task order issued to the Texas A&M center and will enhance protection from anthrax disease. Texas A&M says that under the 18-month, $10.49 million task order from HHS’ Biomedical Advanced Research and Development Authority (BARDA), the Texas A&M CIADM, located in College Station, Texas, will begin advanced development and manufacturing activities for Altimmune’s NasoShield vaccine candidate, a third generation anthrax vaccine.
“Texas A&M has a long tradition of dedicated service to our state and nation,” said John Sharp, chancellor of The Texas A&M University System. “Ultimately, efforts at Texas A&M, including our work on an anthrax vaccine, build upon that tradition and put Texas A&M at the forefront of biosecurity and preparedness, leading the way to a safer future for all.”
The Texas A&M facility is one of three CIADMs — and the only academically-based center — established as public-private partnerships with BARDA in 2012 to enhance the nation’s emergency preparedness against emerging infectious diseases, including pandemic influenza, and chemical, biological, radiological, and nuclear threats. The CIADM is founded on a $285.6 million public-private partnership, including $176.6 million in funding from HHS, with the remainder cost-shared by commercial and academic partners, as well as $40 million from the state of Texas Emerging Technology Fund.
“Texas A&M is committed to achieving global health security and improving the nation’s biodefense,” said Gerald Parker, associate vice president for public health preparedness at Texas A&M Health Science Center. “Forging novel partnerships with the federal government and leveraging expertise of world-leading, innovation-driven commercial partners is one example of Texas A&M’s leadership in service for public health preparedness.”
Texas A&M says that under the task order, the center will partner with Altimmune and engage its subcontractor FUJIFILM Diosynth Biotechnologies Texas, LLC (FDBT) to produce Altimmune’s NasoShield vaccine candidate for use in Phase I clinical trials. This vaccine uses Altimmune’s RespirVec technology, which utilizes a replication-deficient adenovirus to engage the immune system in an innovative way to give protection against anthrax.
“Unlike the only current Food and Drug Administration (FDA)-approved anthrax vaccine that requires several injections and yearly boosters to maintain its protective efficacy, the NasoShield vaccine candidate is being developed because it offers the potential to provide rapid protection against the disease after a single, intranasal dose,” said Scot Roberts, chief scientific officer at Altimmune.
“The potential to have two anthrax vaccines available for the nation’s strategic stockpile, and one based on a novel platform that provides rapid protection after only one dose, will equip public health authorities with new planning options, significantly augmenting biodefense preparedness,” Parker said.
Over the last four years, the Texas A&M center has been designing and building the necessary physical infrastructure, including facilities and manufacturing lines, to provide near-immediate response to support development and manufacturing of life-saving vaccine and medications for the next global public health threat.
“The threat of bioterrorism — one of the most insidious forms of a weapon of mass destruction — is very real and something we as a nation need to be prepared to face so that we can mitigate potential damage and save lives,” Parker said. “As a leading academic institution with a history of dedicated national service, Texas A&M is highly motivated to serve as the bridge between laboratory discovery of anthrax countermeasures and delivery to communities in need of medical tools to fight the infectious disease.”
