MaterialsMother-of-pearl inspires design of stronger materials
In nature, the strength of mother-of-pearl is a key to survival for some shellfish; researchers have offered an explanation for the unusual resilience – and they believes the findings could serve as a blueprint for engineering tough new materials in the laboratory
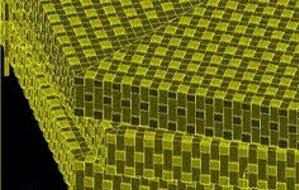
Key to nacre's strength is the interlocking microcrystalline pattern // Source: ndsu.edu
In nature, the strength of mother-of-pearl is a key to survival for some shellfish. Now a team led by Xiaodong Li, an engineering professor at the University of South Carolina, has offered an explanation for the unusual resilience that this defensive shield shows in the face of predatory attacks. Given the elaborate nanoscale structures that biology naturally incorporates in mother-of-pearl, the research team believes the findings could serve as a blueprint for engineering tough new materials in the laboratory.
“For a long time, we’ve thought that we understood how these nanoscale biomaterials work—but it turns out we just know a little bit,” said Li, whose team published their results in a just-released article Nature Publishing’s new journal, Scientific Reports.
A University of South Carolina release reports that mother-of-pearl, also called nacre, makes up the inner shell lining of pearl mussels and some other mollusks.
Pearls themselves are made of nacre, which is a composite nanomaterial constructed by the biomachinery of the shellfish. Tiny crystal grains of calcium carbonate are arranged in a regular, intricate pattern and bound together by biopolymers in nacre’s structure, which adds a tremendous amount of stability to the material: it is some 1,000 times more resistant to cracking from impact than the crystalline form of calcium carbonate (the mineral aragonite) that makes up the bulk of nacre.
Calcium carbonate by itself is best known as blackboard chalk; its tendency to crumble appears to undermine any notion that it would serve as an effective means of stopping a bullet. Yet nature organizes a complex brick-and-mortar-like structure — with the bricks of calcium carbonate measuring in the range of nanometers — to create an incredibly tough material, much stronger than the sum its parts. Mother-of-pearl’s shimmering quality is a by-product of this structure, because the visible light that it reflects has wavelengths that are similar in size to the nanoscale bricks therein.
Nacre’s strength under pressure, Li explained, is unusual and somewhat counter to intuition. When squeezed quickly (dynamic loading), it withstands far more pressure than when squeezed slowly (static loading). “This is a feature of natural materials with nanoparticle architectures,” said Li, “Hardly any man-made ceramics have this property, which would be invaluable in applications like body armor, so understanding how it works is very important.”
The release notes that the increased strength of nacre in the face of rapid pressure has been known for ten years, but the reasons underlying it have remained unclear. So Li’s team set out to understand the mechanism by focusing on the structure of nacre at the nanoscale. They precisely cut mother-of-pearl samples from California red abalone and subjected them to both dynamic and static loading. The nacre that was squeezed rapidly — the ballistic test, in a sense — put up more than twice as much resistance before fracturing than that squeezed slowly. Then Li and co-workers, which included USC researchers as well as contributors from the University of North Carolina at Charlotte, used transmission electron microscopy to address the details of the fracturing at the nanoscale level.
Their results were completely unexpected. Under rapid-compression ballistic conditions, the nanoscale particles work together to restrain the buckling of the material. The researchers concluded that deformation twinning, a process seen in some metals and a particular indicator of strength in the face of stress, comes into play with the nanoscale particles of calcium carbonate. This mechanism, however, was only evident with ballistic conditions, not under the slower application of pressure. Li’s team also concluded that partial dislocations within the nanostructure lend further strength to the material, but again, it only occurred under the ballistic conditions.
When confronted by a short, powerful thrust from a predator — an activity that shellfish have spent many millions of years working out their defense against — the nanostructural bricks in the overall nacre structure work together to absorb the impact and maximize resistance. Stress is first absorbed and dissipated within the nanostructure itself before the material itself is overpowered and fractures.
Now that Li’s team has elucidated the means of mother-of-pearl’s heightened defenses, engineers can try to apply the lessons to synthetic materials. “The real goal is to be able to design these materials,” said Li. “Understanding the mechanism is the first step to making, as just one example, better bullet-proof materials.”
— Read more inZaiwang Huang et al., “Uncovering high-strain rate protection mechanism in nacre,” Scientific Reports 1, Article number 148 (8 November 2011) (doi:10.1038/srep00148)
