-
Klain defends CDC protocols after lab technician’s potential exposure to Ebola
The Obama administration’s Ebola czar, Ron Klain, yesterday (Sunday) defended the security procedures of the Centers for Disease Control and Prevention (CDC), after a technician at one of the agency’s labs in Atlanta was potentially exposed to the deadly disease. The CDC has been criticized earlier this year not only for its response to the Ebola outbreak and Ebola cases within the United States. Numerous safety violations and lax procedures have been reported in the CDC’s labs and in the manner the agency’s technicians transport lethal pathogens, including anthrax and botulism bacteria, from one lab to another.
-
-
Scientists support research which increases microbes’ virulence, transmissibility, or host range
Amid new concerns about lab safety lapses and in a counterpoint to recent calls for restrictions on research that may render pathogens more dangerous, thirty-six scientists from several countries have issued a formal statement asserting that research on potentially dangerous pathogens can be done safely, and is necessary for a full understanding of infectious diseases. The statement rejects calls for limiting “gain-of-function” (GOF) research, that is, experiments which involve increasing the virulence, transmissibility, or host range of microbes.
-
-
Research institutions must support strong, positive safety culture in chemical labs
Everyone involved in the academic chemical research enterprise — from researchers and principal investigators to university leadership — has an important role to play in establishing and promoting a strong, positive safety culture, says a new report from the National Research Council. This requires a constant commitment to safety organization-wide and emphasis on identifying and solving problems, rather than merely adhering to a set of rules and assigning blame when those rules are not followed.
-
-
CDC resumes pathogen shipments
Last Thursday, the Centers for Disease Control and Prevention(CDC) announced it would reopen its clinical tuberculosis lab to resume transfer of inactivated tuberculosis bacteria to lower-level CDC labs for genetic analysis. CDC head Tom Frieden imposed a ban on transfers involving high-level pathogens following a series of incidents and mishandling of such pathogens at CDC labs.
-
-
Lawmaker says CDC made false lab safety pledges
A house panel is investigating repeated safety lapses at key government laboratories, including an incident in which eighty lab workers were likely exposed to live anthrax bacteria at an Atlanta facility. The group is also investigating the CDC’s responses to the incidents. The committee chairman noted that CDC had in the past offered assurances that it was tightening monitoring of labs’ safety procedures, but that such pledges were not fulfilled.
-
-
The number of labs handling deadly germs grows, and so do calls for regulating lab safety
The number of labs handling dangerous pathogens continues to grow, and so does the number of accidents involving dangerous pathogens. The number of reported accidents involving dangerous microbes grew rapidly from just sixteen in 2004 to 128 in 2008, and 269 in 2010, the last year reported.Experts note that currently there is no single federal agency responsible for assessing overall laboratory needs — instead, departments and agencies only assess the needs for labs relative to their respective missions.
-
-
Concerns grow about CDC’s tracking, securing dangerous pathogens under its supervision
Last week, Centers for Disease Control and Prevention(CDC) officials reported that the same federal scientist who found vials of smallpox in a Food and Drug Administration(FDA) cold storage room at the National Institutes of Healthfacility in Bethesda, Maryland, also found a collection of 327 vials which could contain pathogens like dengue, influenza, and rickettsia. The new revelation adds to growing concerns about the government’s ability to track and secure dangerous pathogens under its supervision.”It is ironic that the institution that sets U.S. standards for safety and security of work with human pathogens fails to meet its own standards,” says a security expert. “It is clear that the CDC cannot be relied upon to police its own select-agent labs.”
-
-
Head of biosecurity advisory panel: Board is stalling as a result of slow fed policy work
The head of a federal biosecurity advisory committee says delays in the development of a national policy on institutional oversight of risky life-sciences research are the main reason the committee has been inactive for close to two years. The dormancy of the National Science Advisory Board for Biosecurity (NSABB) was pushed into the spotlight this week with the revelation that the eleven remaining original members of the 23-member board are being replaced. The board was set up in 2005 to advise the government on biosecurity and dual-use research, meaning research that can be exploited for harm as well as good.
-
-
Investigation finds serious violations of safety rules in CDC’s handling of deadly germs
An investigation by the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service(APHIS) conducted a review, from 23 June to 3 July, of the Centers for Disease Control and Prevention(CDC), and cited the agency for failing to follow proper procedures before and after the anthrax scare which led to the potential exposure of more than eighty lab workers to live anthrax viruses in June.APHIS found multiple violations of federal rules for handling dangerous microbes.
-
-
Following accidents, CDC shuts down anthrax, flu labs
Federal officials announced on Friday that they had temporarily closed the flu and anthrax laboratories at the Centers for Disease Control and Prevention (CDC) in Atlanta and halted shipments of all infectious agents from the agency’s highest-security labs. The announcement followed revelations about two recent accidents involving deadly agents at the CDC campus in Atlanta. Critics said the accidents highlighted an even greater danger – the efforts at some labs to create superstrains of deadly viruses (what is called “gain of function” research). “You can have all the safety procedures in the world, but you can’t provide for human error,” a critic of gain-of-function research said.
-
-
NIH employees not notified of smallpox virus vials found at NIH Md. campus
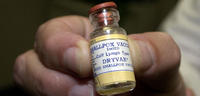
When Food and Drug Administration(FDA) workers the other day discovered decades-old vials of smallpox virus in Building 29A on the Bethesda, Maryland campus of the National Institutes of Health(NIH), NIH officials reached out to Montgomery County officials, Maryland health officials, and senior NIH executives.No notification, however, was sent to the roughly 18,000 NIH employees who work at the agency’s main campus.
-
-
Smallpox vials found unguarded at NIH campus in Bethesda, Md.
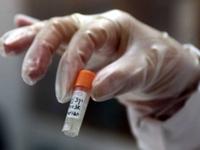
Earlier this month workers clearing out a Food and Drug Administration(FDA) branch office at the National Institutes of Health(NIH) campus in Bethesda, Maryland, discovered vials containing smallpox, an eradicated agent feared for its bioweapons potential. The last smallpox samples in existence were thought to be held at tightly guarded facilities in Atlanta and the State Research Center of Virology and Biotechnologyin Novosibirsk, Russia. The vials appear to date from the 1950s.
-
-
CDC says anthrax infection “highly unlikely,” but reassigns bioterror lab chief
The U.S. Centers for Disease Control and Prevention(CDC) has advised some of its employees to stop taking antibiotics meant to fight a possible anthrax infection after preliminary tests suggest that it is “highly unlikely” those employees were exposed to live anthrax following an incident in June. Michael Farrell, head of the CDC bioterror lab, has been reassigned.
-
-
Canada donates Biosafety Level 3 modular laboratory to Caribbean health authorities
The Biological Security program of Canada’s Global Partnership Program(GPP) has officially transferred a new biological containment laboratory to the Caribbean Public Health Agency(CARPHA). The Biosafety Level 3 (BSL-3) modular laboratory facility, a first in the Caribbean and located in Port of Spain, Trinidad and Tobago, improves diagnostic capabilities for human and veterinary pathogens with high epidemic potential.
-
-
Leidos awarded DHS Plum Island biolab contract
DHS awarded Reston, Virginia-based Leidos a prime contract to support and supplement the Science and Technology (S&T) Agricultural Scientific Program at the Plum Island Animal Disease Center (PIADC). The single-award time and materials (T&M) contract has a one-year base period of performance, four one-year options, and a total contract value of approximately $12 million if all options are exercised. Work will be performed in Orient Point, New York.
-
- All
- Regional
- Water
- Biometrics
- Borders/Immig
- Business
- Cybersecurity
- Detection
- Disasters
- Government
- Infrastructure
- International
- Public health
- Public Safety
- Communication interoperabillity
- Emergency services
- Emergency medical services
- Fire
- First response
- IEDs
- Law Enforcement
- Law Enforcement Technology
- Military technology
- Nonlethal weapons
- Nuclear weapons
- Personal protection equipment
- Police
- Notification /alert systems
- Situational awareness
- Weapons systems
- Sci-Tech
- Sector Reports
- Surveillance
- Transportation
Advertising & Marketing: advertise@newswirepubs.com
Editorial: editor@newswirepubs.com
General: info@newswirepubs.com
2010-2011 © News Wire Publications, LLC News Wire Publications, LLC
220 Old Country Road | Suite 200 | Mineola | New York | 11501
Permissions and Policies
Editorial: editor@newswirepubs.com
General: info@newswirepubs.com
2010-2011 © News Wire Publications, LLC News Wire Publications, LLC
220 Old Country Road | Suite 200 | Mineola | New York | 11501
Permissions and Policies
