-
Funding for broad spectrum prophylaxis, treatment for bioterrorism threats
The U.K. Defense Science and Technology Laboratory (DSTL) has received funding of up to $6.9 million from the U.S. Defense Threat Reduction Agency (DTRA) for a program entitled “Inhalational ciprofloxacin for improved protection against biowarfare agents.” The inhalational ciprofloxacin formulations used in this program are Aradigm’s proprietary investigational drugs Pulmaquin and Lipoquin.
-
-
New candidate vaccines against the plague show promise
The plague of Black Death infamy has had the power to strike fear in people since the Middle Ages — and for good reason. Once someone begins to show symptoms, the disease progresses very quickly and is almost 100 percent fatal without prompt treatment. Antibiotic-resistant Y. pestis strains have been isolated from plague patients and can be engineered for use as a bioweapon. Researchers have developed new potential vaccines that protect animals against the bacteria that causes the deadly plague.
-
-
Assessing the risk from Africa as Libya loses its chemical weapons
Libya’s remaining chemical weapons left over from the Gaddafi regime are now being safely disposed of in a German facility. This eliminates the risk of them falling into the wrong hands. But can these same hands acquire weapons of mass destruction from the rest of Africa? The disposal of Libya’s chemical weapons has lowered the risk of weapons of mass destruction in Africa. But we have seen how far non-state actors are willing to go to either produce or steal such weapons. For example, analysts envision militants known as “suicide infectors” visiting an area with an infectious disease outbreak like Ebola purposely to infect themselves and then using air travel to carry out the attack. Reports from 2009 show forty al-Qaeda linked militants being killed by the plague at a training camp in Algeria. There were claims that they were developing the disease as a weapon. The threat WMD pose cannot be ignored. African countries, with help from bilateral partners and the international community, have broadened their nonproliferation focus. They will need to keep doing so if the goal is effectively to counter this threat.
-
-
Building a biosafety and biosecurity toolkit for a safer gene editing research
A new DARPA program could help unlock the potential of advanced gene editing technologies by developing a set of tools to address potential risks of this rapidly advancing field. The Safe Genes program envisions addressing key safety gaps by using those tools to restrict or reverse the propagation of engineered genetic constructs. Safe Genes was inspired in part by recent advances in the field of “gene drives,” which can alter the genetic character of a population of organisms by ensuring that certain edited genetic traits are passed down to almost every individual in subsequent generations.
-
-
Growing concern about amateur “biohackers” creating biological weapons
American and European security agencies have been increasingly focusing on the risk that “biohackers” – scientists who use genome-editing techniques to change life forms by increasing or decreasing the function of genes — could develop biological weapons or other dangerous biological substances. The problem is not only – or even mostly – with the work of professional scientists. Rather, the real danger lies with amateur scientists around the world who have started to use gene-editing techniques after the tools became cheap and readily available.
-
-
Keeping pace with the fast-developing science of gene drives
The emerging science of gene drives is drawing attention for its potential to help with critical health issues such as mosquito-borne diseases and environmental concerns such as agricultural pests and invasive species. At its most basic, a gene drive operates outside the traditional realm of genetics, in which an offspring has a 50-50 chance of inheriting a trait from one of its parents. A gene drive introduces a trait that will spread — or drive — through a population. “The science of gene drives is moving very fast,” says an expert. “[O]ur ability to assess the risks of gene drives, to oversee them with regulatory agencies, and to have a public discussion around gene drives is falling behind the science — We don’t want to wait until we have the technology in front of us to have discussions about regulation, oversight, ethics, and engagement.”
-
-
FBI’s WMD Directorate marks its first decade
If you can imagine a disaster involving explosives or the release of nuclear, biological, chemical, or radioactive material, there is a pretty good chance a group of subject-matter experts within the FBI has built an elaborate scenario around it and tested how well emergency responders face up to it. It is the main jobs of the Weapons of Mass Destruction (WMD) Directorate — to imagine worst-case scenarios and then devise ways to prevent and prepare for them. The Directorate was created ten years ago, on 26 July 2006.
-
-
Using gels for biological decontamination

Removing chemical, biological, radiological, and toxic contaminants from a range of surface types could be as easy as peeling off a sticker thanks to research conducted by scientists at the U.S. Army’s Edgewood Chemical Biological Center (ECBC) and industry partner CBI Polymer.The researchers explored how a HydroGel can be modified to decontaminate surfaces contaminated with biological agents.
-
-
Anthrax capsule vaccine completely protects monkeys from lethal inhalational anthrax
Vaccination with the anthrax capsule — a naturally occurring component of the bacterium that causes the disease — completely protected monkeys from lethal anthrax infection, according to a new study. These results indicate that anthrax capsule is a highly effective vaccine component that should be considered for incorporation in future generation anthrax vaccines.
-
-
FDA completes pre-approval inspection of Emergent BioSolutions’ anthrax vaccine manufacturing facility
Gaithersburg, Maryland-based Emergent BioSolutions Inc. last week announced that the U.S. Food and Drug Administration (FDA) had completed its Pre-Approval Inspection (PAI) of Building 55, the company’s facility for large-scale manufacturing of BioThrax (Anthrax Vaccine Adsorbed).
-
-
Testing NYC subway biodefenses

Researchers took to the New York City subway system 9-13 May to study how a surrogate for a biological agent, such as anthrax, might disperse throughout the nation’s largest rapid transit system as a result of a terrorist attack or an accidental release. The study is part of a five-year DHS project called Underground Transport Restoration (UTR) and was conducted in accordance with the National Environmental Policy Act.
-
-
Biodefense Panel welcomes key provision in defense authorization bill
In October 2015, the Blue Ribbon Study Panel on Biodefense found that insufficient federal coordination on strategy, budgeting, and policy; inadequate collaboration with other levels of government and the private sector; and lagging innovation in areas like biosurveillance and medical countermeasure development make the United States vulnerable to biological attacks and infectious disease outbreaks. The Panel welcomed the passing by the House of the National Defense Authorization Act, H.R. 4909, which includes a provision addressing one of the Panel’s most important recommendations.
-
-
Developing new anthrax vaccine
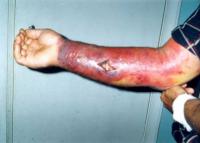
The Texas A&M Center for Innovation in Advanced Development and Manufacturing (CIADM) will produce an intranasal anthrax vaccine candidate under a task order issued by the U.S. Department of Health and Human Services (HHS). This is the first task order issued to the Texas A&M center and will enhance protection from anthrax disease. The Texas A&M facility is one of three CIADMs — and the only academically-based center — established as public-private partnerships with BARDA in 2012 to enhance the nation’s emergency preparedness against emerging infectious diseases.
-
-
Airflow study to be conducted in NYC Subway
The Department of Homeland Security (DHS) Science and Technology Directorate (S&T) will conduct a week-long airflow study in portions of the New York City (NYC) subway system to gather data on the behavior of airborne particles in the event contaminants were released. This study poses no risk to the general public and will run from 9 to 13 May.
-
-
New Yorker sentenced to 16 years for trying to buy ricin
It was a scary scenario: Chinese national Cheng Le, living in New York City, attempted to order ricin through the so-called dark Web. Ricin is a highly potent and potentially fatal toxin with no known antidote. What did Le plan to do with the ricin? Nothing good. According to U.S. Attorney for the Southern District of New York Preet Bharara, “In Le’s own words, established at trial, he was looking for ‘simple and easy death pills’ and ways to commit ‘100 percent risk-free’ murder.”
-
- All
- Regional
- Water
- Biometrics
- Borders/Immig
- Business
- Cybersecurity
- Detection
- Disasters
- Government
- Infrastructure
- International
- Public health
- Public Safety
- Communication interoperabillity
- Emergency services
- Emergency medical services
- Fire
- First response
- IEDs
- Law Enforcement
- Law Enforcement Technology
- Military technology
- Nonlethal weapons
- Nuclear weapons
- Personal protection equipment
- Police
- Notification /alert systems
- Situational awareness
- Weapons systems
- Sci-Tech
- Sector Reports
- Surveillance
- Transportation
Advertising & Marketing: advertise@newswirepubs.com
Editorial: editor@newswirepubs.com
General: info@newswirepubs.com
2010-2011 © News Wire Publications, LLC News Wire Publications, LLC
220 Old Country Road | Suite 200 | Mineola | New York | 11501
Permissions and Policies
Editorial: editor@newswirepubs.com
General: info@newswirepubs.com
2010-2011 © News Wire Publications, LLC News Wire Publications, LLC
220 Old Country Road | Suite 200 | Mineola | New York | 11501
Permissions and Policies
