-
Unvaccinated adults cost the U.S. economy more than $7 billion a year
Vaccine-preventable diseases among adults cost the U.S. economy $8.95 billion in 2015, and unvaccinated individuals are responsible for 80 percent, or $7.1 billion, of the tab. The flu was the most costly disease with a vaccine available, accounting for nearly $5.8 billion in health care costs and lost productivity in 2015.
-
-
Funding for broad spectrum prophylaxis, treatment for bioterrorism threats
The U.K. Defense Science and Technology Laboratory (DSTL) has received funding of up to $6.9 million from the U.S. Defense Threat Reduction Agency (DTRA) for a program entitled “Inhalational ciprofloxacin for improved protection against biowarfare agents.” The inhalational ciprofloxacin formulations used in this program are Aradigm’s proprietary investigational drugs Pulmaquin and Lipoquin.
-
-
Texas must reduce nonmedical exemptions to vaccinations
In Texas, approximately 45,000 nonmedical exemptions were filed across all age groups during the 2015-16 school year, a record high in the last decade and a figure that is only increasing. Vaccines are one of most cost-effective public health measures, the authors of a new study write, and Texas should make the process of obtaining nonmedical exemptions more rigorous to avoid the public health risks and costs associated with preventable diseases.
-
-
New candidate vaccines against the plague show promise
The plague of Black Death infamy has had the power to strike fear in people since the Middle Ages — and for good reason. Once someone begins to show symptoms, the disease progresses very quickly and is almost 100 percent fatal without prompt treatment. Antibiotic-resistant Y. pestis strains have been isolated from plague patients and can be engineered for use as a bioweapon. Researchers have developed new potential vaccines that protect animals against the bacteria that causes the deadly plague.
-
-
Star-shaped polymers, not antibiotics, kill antibiotic-resistant bacteria
Currently, the only treatment for infections caused by bacteria is antibiotics. However, over time bacteria mutate to protect themselves against antibiotics, making treatment no longer effective. These mutated bacteria are known as “superbugs.” Tiny, star-shaped molecules are effective at killing bacteria that can no longer be killed by current antibiotics, new research shows. The research holds promise for a new treatment method against antibiotic-resistant bacteria, or superbugs.
-
-
Vaccine against “flesh-eating” bacteria in sight
Biochemists have uncovered patterns in the outer protein coat of group A Streptococcus that could finally lead to a vaccine against this highly infectious bacteria — responsible for more than 500,000 deaths a year, including toxic shock syndrome and necrotizing fasciitis or “flesh-eating disease.”
-
-
Climate, air travel maps identify countries in Africa, Asia at greatest risk of Zika virus
Many countries across Africa and Asia-Pacific may be vulnerable to Zika virus outbreaks, with India, China, the Philippines, Indonesia, Nigeria, Vietnam, Pakistan, and Bangladesh expected to be at greatest risk of Zika virus transmission due to a combination of high travel volumes from Zika affected areas in the Americas, local presence of mosquitos capable of transmitting Zika virus, suitable climatic conditions, large populations, and limited health resources. The authors of a new study say that identifying where and when populations would be most susceptible to local transmission of Zika virus could help inform public health decisions about the use of finite resources.
-
-
Zika reference strain sequenced; will help in diagnosis, screening
An international team of researchers has sequenced a strain of the Zika virus that will be used as a World Health Organization (WHO) reference strain to identify Zika virus infection in the blood, thus making it easier to diagnose the disease.
-
-
$2.3 million grant for Ebola vaccine research
The Center of Excellence for Emerging Zoonotic and Animal Diseases, or CEEZAD, at Kansas State University will use a $2.3 million federal grant to study the safety in livestock of a newly developed vaccine to protect humans from the Ebola Zaire virus. No infectious Ebola virus will be used in the Biosecurity Research Institute during the studies.
-
-
GMOs lead the fight against Zika, Ebola and the next unknown pandemic
The shadow of the Zika virus hangs over the Rio Olympic Games, with visitors and even high-profile athletes citing worries about Zika as a reason to stay away (even if the risk is probably quite low). The public’s concerns are a striking example of the need to rapidly combat emerging infectious diseases. In the fight against Zika, public health experts have turned to what may sound like an unlikely ally: genetically modified organisms, or GMOs. To protect the public, scientists have embraced GMO technology to quickly study new health threats, manufacture enough protective vaccines, and monitor and even predict new outbreaks. With the help of GMOs, infectious disease experts have the tools to get ahead of the next outbreak, moving beyond reaction to quick detection, containment and even prevention.
-
-
Anthrax capsule vaccine completely protects monkeys from lethal inhalational anthrax
Vaccination with the anthrax capsule — a naturally occurring component of the bacterium that causes the disease — completely protected monkeys from lethal anthrax infection, according to a new study. These results indicate that anthrax capsule is a highly effective vaccine component that should be considered for incorporation in future generation anthrax vaccines.
-
-
Infectious outbreaks must be combatted strategically: Experts
New funding is not enough to guarantee success against emerging infectious diseases around the world. Rather, good governance, a long-term technology investment strategy, and strong product management skills are essential. As momentum builds for an international effort to develop drugs and vaccines for emerging infectious diseases, experts examine U.S. biodefense programs to understand approaches that might work and developed a global strategy for countermeasure development.
-
-
Stagnant U.S. funding for tools against infectious diseases leaves U.S., world at serious risk
As Congress grapples with the White House on how to fund an emergency response to fight Zika virus, a new report warns that overall underfunding for development of lifesaving tools against neglected global diseases is putting the United States and the world at risk, and that emergency funding cannot be allowed to substitute for sustained U.S. investment in research and development (R&D) of global health technologies. A recent study that examined the risk of infectious disease outbreaks projected that large-scale global disease pandemics could cost the global economy more than $60 billion a year, while investing in the interventions needed to protect against these outbreaks, including R&D, would cost only a fraction of that — $4.5 billion — each year.
-
-
Resistance-proof antiviral can treat many diseases
Scientists and health officials are marshalling forces to fight Zika, the latest in a string of recent outbreaks. Many of these efforts target that virus specifically, but some researchers are looking for a broader approach. The new strategy aims to fight a wide range of viruses that appears to be safe in vivo and could evade a virus’s ability to develop resistance.
-
-
Most vaccine-related posts on Pinterest are anti-vaccine: Study
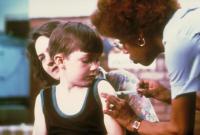
About 75 percent of the vaccine-related posts on Pinterest are negative toward vaccination, according to new research. Messages ranged from simple posts questioning the safety of vaccines to more radical claims that vaccines are being created to kill people. Twenty percent of the posts talked about conspiracy theories, such as pharmaceutical companies out to make money at the expense of children and governments trying to harm children for the purposes of population control. The authors are calling for better communication about vaccination.
-
More headlines
The long view
A Shining Star in a Contentious Legacy: Could Marty Makary Be the Saving Grace of a Divisive Presidency?
While much of the Trump administration has sparked controversy, the FDA’s consumer-first reforms may be remembered as its brightest legacy. From AI-driven drug reviews to bans on artificial dyes, the FDA’s agenda resonates with the public in ways few Trump-era policies have.
