Fuel cellsMaking cleaner fuel cells
There is a near unanimity in the scientific community that the average temperature on the planet is rising and this is happening because of the increased concentration of carbon dioxide in the atmosphere. Radically redesigning virtually all technological infrastructure is not possible without an acceptable alternative to internal combustion engines: either electric accumulators and electric motors, or fuel cells with electric motors. Fuel cells themselves will not solve the problem of rising temperatures on the planet, but they are part of a possible solution. Researchers have developed ion-exchange synthetic membranes based on amphiphilic compounds that are able to convert the energy of chemical reactions into electrical current.
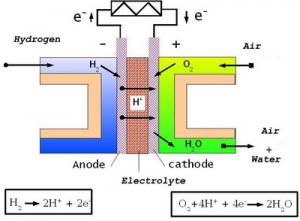
Schematic diagram of currently fuel cell // Source: commons.wikimedia.org
An international group of scientists from Russia, France, and Germany have developed ion-exchange synthetic membranes based on amphiphilic compounds which are able to convert the energy of chemical reactions into electrical current. The new development described in the journal Physical Chemistry, Chemical Physics could potentially be used in fuel cells, and in separation and purification processes. The study was conducted by Moscow Institute of Physics and Technology’s (MIPT) Laboratory of Functional Organic and Hybrid Materials, which was opened in 2014.
Fuel cells consist of separate galvanic cells and their closest relatives are batteries (primary cells) and accumulators (secondary cells). Batteries convert the energy of the reaction between an oxidizing agent and a reducing agent, and stop working when these agents are used up. An accumulator is able to store electrical energy applied to it from an external source, convert it to chemical energy, and release it again, thus reversing the process. A fuel cell, on the other hand, which is also an electrochemical generator, gets the materials that it needs to function from an external source. These materials are a reducing agent (usually hydrogen, methanol or methane) and an oxidizing agent, oxygen. Providing these materials from an external source means that electricity can be obtained from a fuel cell continuously without having to stop to recharge for as long as the parts of the cell are in working order.
The main elements of this generator are a cathode and an anode, separated by an ion-exchange membrane.
At the cathode, the reducing agent is dissociated — an electron is separated from a hydrogen molecule (or another fuel) and thus a positively charged hydrogen ion, a proton, is formed. The membrane allows protons to pass through, but retains the electrons — these particles are forced to take the “long route” through an external circuit. Only once they have passed through this circuit (the device that the fuel cell is powering) can they reach the anode where they find oxygen and the protons that passed through the membrane to combine and form water. The electrons, which are forced to go around the membrane, create a current in the external circuit that can be utilized.
